Ionic Liquids & e-SILP
Custom-Tailored Ionic Liquids & Ionic Liquid Crystals for Interface-Enhanced e-SILP Catalysis
Ionic Liquids (ILs), generally defined as salts with melting points below 100 °C, are composed of discrete ions, which can be of organic or inorganic nature. Due to the wide variety of suitable anion-cation combinations, ILs have swiftly tunable physical and chemical properties. This results in a unique property profile, including negligible vapor pressure and non-flammability, wide liquid ranges, electric conductivities and wide electrochemical windows, as well as high thermal stability, density and viscosity. These properties provide many possibilities for application as “green” solvents, electrolytes or in industrial processes.
Moreover, ILs can feature a liquid crystalline state, combining the properties of ionic liquids and liquid crystals simultaneously; that means, so-called ionic liquid crystals (ILCs) provide e.g. liquidity over a wide temperature range and high electric conductivity as well as dynamic molecular order and anisotropic physical properties. Hence, these compounds are well suitable i.a. as pre-organized reaction media for selective catalysis or as anisotropic ion-conductive materials.
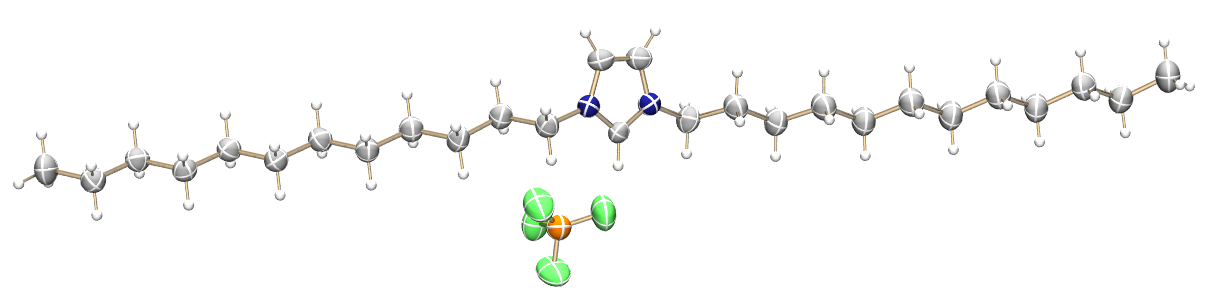
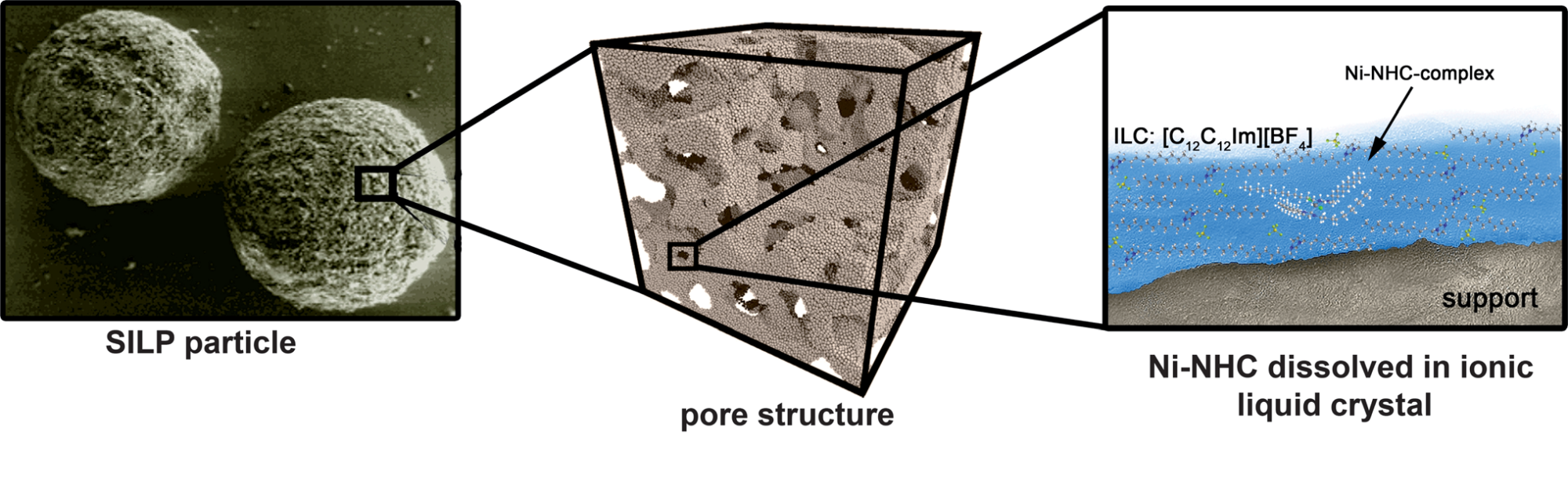
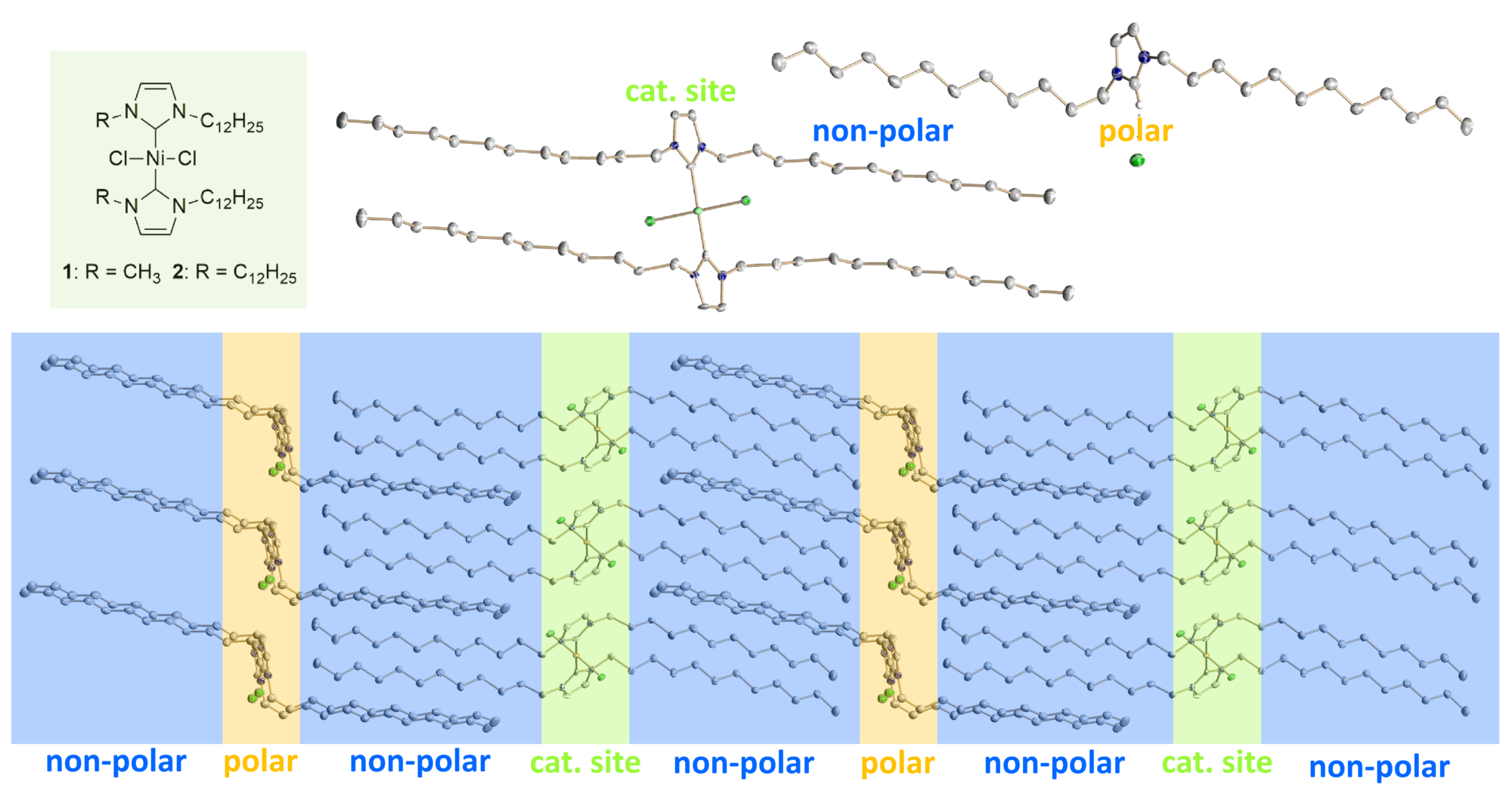
In this context, the Meyer group has focused on the development of long alkyl chain functionalized imidazolium cations. These ILs exhibit ILC phases at or near room temperature and thus, are suitable as solvents for liquid crystal supported ionic liquid phase (LC-SILP) catalysis for olefin dimerization. Within this attempt, an appropriate NiII catalyst and the ILC were both immobilized on a solid support combining the advantages of heterogeneous and homogeneous catalysis.
Most recent projects comprise the development of less hygroscopic long alkyl chain substituted imidazolinium-based ILs as well as chiral ILs and ILCs for enantioselective catalysis. In addition, we place special emphasis on the development of hydrophilic ether substituted ILs as well as on the synthesis of asymmetric substituted ILs, which can be used for immobilization on a solid support via a linker entity.
For details on our actinide chemistry program, please check out the following selection of references:
X. Wang, F. W. Heinemann, M. Yang, B. U. Melcher, M. Fekete, A.-V. Mudring, P. Wasserscheid and K. Meyer
A new class of double alkyl-substituted, liquid crystalline imidazolium ionic liquids—a unique combination of structural features, viscosity effects, and thermal properties
Chem. Commun. 2009, 7405–7407.
X. Wang, C. S. Vogel, F. W. Heinemann, P. Wasserscheid and K. Meyer
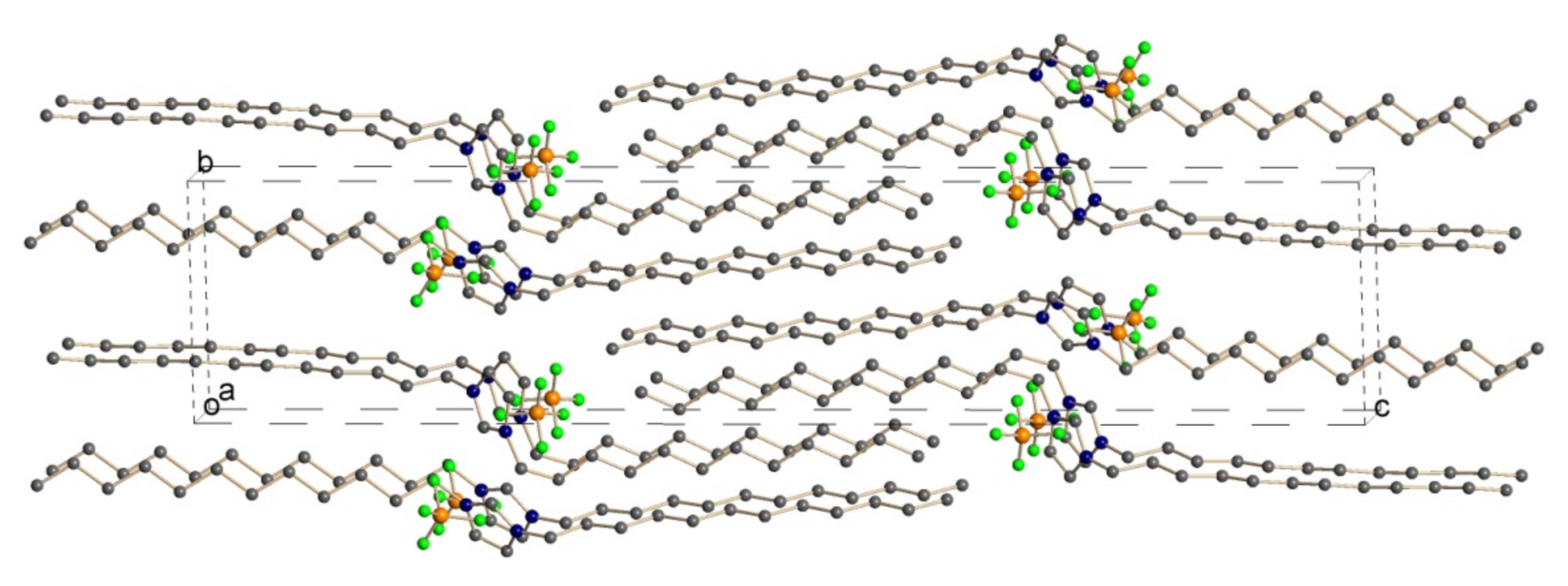
Solid-State Structures of Double-Long-Chain Imidazolium Ionic Liquids: Influence of Anion Shape on Cation Geometry and Crystal Packing
Cryst. Growth Des. 2011, 11, 1974–1988.
X. Wang, M. Sobota, F. T. U. Kohler, B. Morain, B. U. Melcher, M. Laurin, P. Wasserscheid, J. Libuda and K. Meyer
Functional nickel complexes of N-heterocyclic carbene ligands in pre-organized and supported thin film materials
J. Mater. Chem. 2012, 22, 1893–1898.
X. Wang, M. Sternberg, F. T. U. Kohler, B. U. Melcher, P. Wasserscheid and K. Meyer
Long-alkyl-chain-derivatized imidazolium salts and ionic liquid crystals with tailor-made properties
RSC Adv. 2014, 4, 12476–12481.
A. Lennert, M. Sternberg, K. Meyer, R. D. Costa and D. M. Guldi
Iodine-pseudohalogen ionic liquid-based electrolytes for quasi-solid-state dy-sensitized solar cells
ACS Appl Mater. Interfaces 2017, 9, 33437–33445.
X. Wang, M. Valldor, E. T. Spielberg, F. W. Heinemann, K. Meyer and A.-V. Mudring
Paramagnetic iron-containing ionic liquid crystals
J. Mol. Liq. 2020, 304, 112583.